Adding the Drugs Tazemetostat or Zanubrutinib to Usual Treatment for Large B-Cell Lymphoma That Returned or Did Not Respond to Previous Treatment
What is the purpose of this clinical trial?
|
This study is for people with certain types of large B-cell lymphoma (LBCL). It will test 2 study drugs for treating lymphoma that came back or did not respond to previous treatment. Researchers will test adding each study drug to a usual treatment that is already used for LBCL. They will compare each of the study treatments to the usual treatment alone. |
Lea la version en español. |
The usual treatment uses 2 drugs:
- tafasitamab (Monjuvi) and
- lenalidomide (Revlimid)
The study treatments combine the usual treatment with one of the study drugs:
- tazemetostat (Tazverik) OR
- zanubrutinib (Brukinsa)
The study drugs tazemetostat and zanubrutinib are both approved by the Food and Drug Administration (FDA) for treating some types of lymphoma.
This trial is set up to find out:
- The right dose (amount) of the study drugs to use with the usual treatment
- If adding either of the study drugs to usual treatment can lower the chance of LBCL getting worse
- If people who have specific changes in their cancer cells are more likely to benefit from tazemetostat or zanubrutinib
Why is this trial important?
This trial is a chance to improve treatment options for lymphoma that came back or did not respond to previous treatment.
The trial may also help doctors learn about using certain information in cancer cells to match patients with treatments. Each of the study drugs being tested (tazemetostat and zanubrutinib) targets cancer differently by blocking certain proteins in cells. If the treatments work, the trial could help doctors know which patients in the future might benefit most from each drug.
Who can be in this trial?
This trial is for adults, age 18 or older, with large B-cell lymphoma, high grade B-cell lymphoma, transformed lymphoma, or follicular lymphoma.
This trial may be for people who:
- Have lymphoma that returned after treatment or did not respond to previous treatment
- May have had a stem cell transplant or CAR T-cell therapy in previous treatment
This trial is not for people who:
- Will have a stem cell transplant
- Already had treatment with any drugs used in this study
- Are pregnant
Talk with your doctor to learn more about who can join this study.
What treatments will I get?
This study has 2 parts. If you join the trial, you will be in Part 1 or Part 2.
Part 1
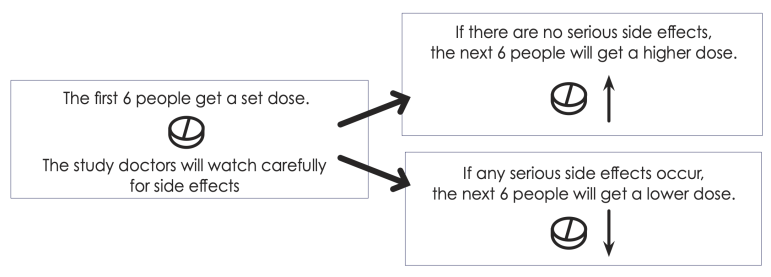
The goal of Part 1 is to find the safest dose of each study drug when it is combined with the usual treatment. The first 24 people in the study will be in Part 1.
If you are in Part 1, your doctor will assign you to get one of 2 study treatments:
Part 1 will test 2 doses of each study drug. Ask your doctor which dose you may get.
If the safest dose is different than the dose you received, your dose may be changed to the safest dose.
Part 2
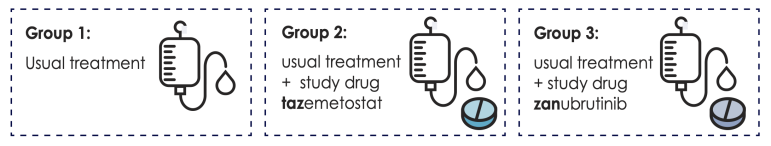
If you are in Part 2, a computer will randomly assign you to one of 3 study groups. Treatments in Part 2 will use the safest dose of each study drug that was found in Part 1.
You will have an equal chance of being in Group 1, Group 2, or Group 3. Your doctor will not have control over which group you will be assigned to. This helps make sure the study results are fair and reliable.
How long will I be in the trial?
In total, you will be in the study for 3 years.
You may get up to 13 cycles of treatment in the study. This will take about 1 year to complete.
After treatment, you will have follow-up visits with the study team until 3 years after you started the study.
Are there costs? Will I get paid?
The drugs tafasitamab, tazemetostat, and zanubrutinib are provided free in the study. Ask your health care provider and insurance provider about what costs will and won’t be covered in this study. You will not be paid for joining the study.
Where can I find more information about this trial?
- Talk with your health care provider
- Call the National Cancer Institute at 1-800-4-CANCER
- Go to www.ClinicalTrials.gov and search using the national clinical trial number: NCT05890352
- For a list of trial locations, visit swog.org/NCI-S2207